ARTI has three projects:
1.
Ferroptosis resistance as a key driver in acquired radiation resistance
2.
Tumor hypoxia promotes acquired resistance to radiation through ferroptosis inhibition
3.
Role of genomic and microenvironment factors in conferring acquired resistance to ferroptosis to chemoradiation in esophageal adenocarcinoma
Hypothesis:
(i) ferroptosis resistance represents a key mechanism underlying acquired radio-resistance in lung and esophageal cancers and (ii) combining FINs with immunotherapy is an effective therapeutic strategy to overcome acquired radio-resistance without causing significant damage in normal tissues.
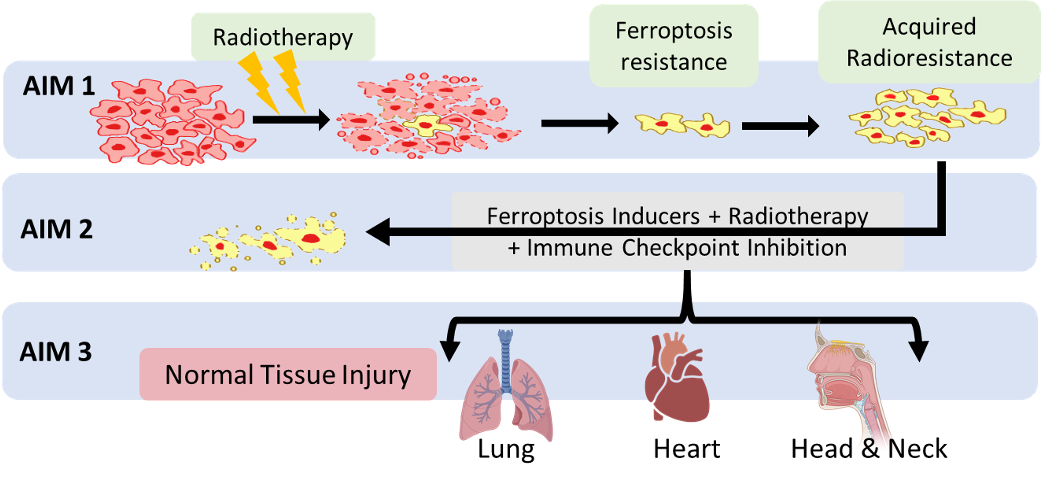
Specific Aim 1:
To define the mechanisms by which ferroptosis resistance drives acquired radio-resistance.
Specific Aim 2:
To determine the effectiveness of combining FINs with immunotherapy in overcoming acquired tumor radio-resistance.
Specific Aim 3:
To determine the potential effects of FINs on radiation-induced toxicity in normal cells and tissues.
(For additional information, please visit NIH RePORTER)
Hypothesis:
(i) hypoxia-induced resistance to ferroptosis contributes to acquired tumor resistance to radiation therapy and (ii) ferroptosis inducers (FINs) may re-sensitize hypoxic tumor cells to radiation treatment.
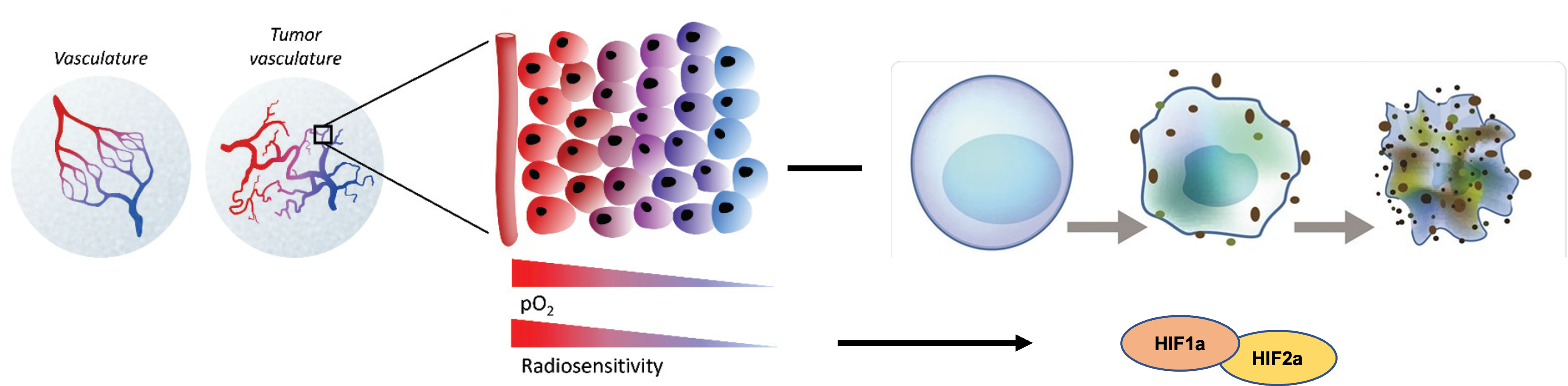
Specific Aim 1:
To demonstrate that hypoxia drives acquired resistance to radiation-induced ferroptosis in vitro.
Specific Aim 2:
To determine the role of HIF1/2alpha in resistance to radiation-induced ferroptosis under hypoxia.
Specific Aim 3:
To determine effect of ferroptosis inducers (FINs) in overcoming acquired tumor radio-resistance induced by hypoxia.
(For additional information, please visit NIH RePORTER)
Hypothesis:
Adaptive resistance to ferroptosis is mediated through intratumoral and microenvironmental heterogeneity that lead to adaptive resistance during chemoradiation in esophageal adenocarcinomas.
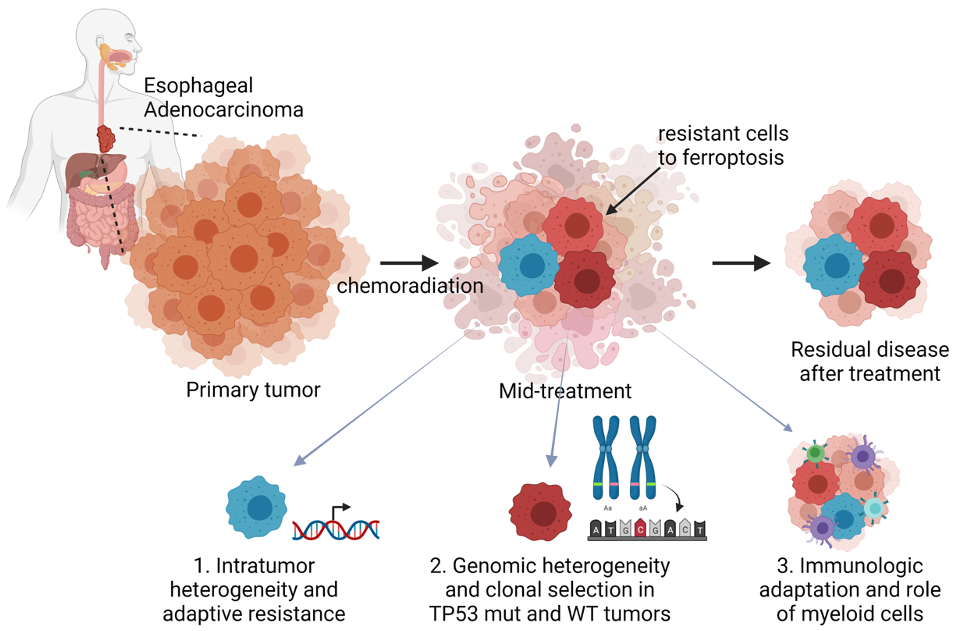
Specific Aim 1:
To evaluate the role of intratumoral heterogeneity in adaptive resistance to ferroptosis in esophageal adenocarcinoma undergoing CRT.
Specific Aim 2:
To examine the role of genomic heterogeneity and clonal selection in conferring chemoradiation therapy resistance to ferroptosis in esophageal adenocarcinoma.
Specific Aim 3:
To assess the role of the adaptive immune response and myeloid cell expansion in ferroptosis resistance.
(For additional information, please visit NIH RePORTER)